Full-colour perovskite LEDs made easy
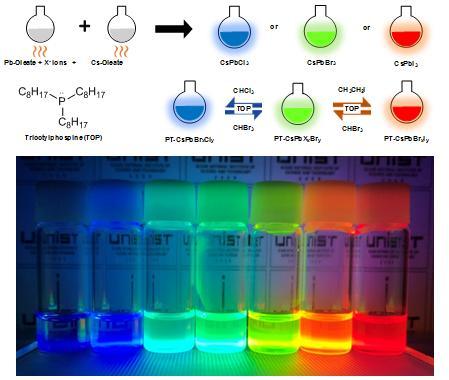
A recent study by scientists at UNIST (Ulsan National Institute of Science and Technology in Korea) has introduced a simple technique to extract the three primary colours (red, blue, green) from perovskite nanoparticles.
This breakthrough has been led by Jin Young Kim in the School of Energy and Chemical Engineering at UNIST. In the study, the research team introduced a simple technique that freely controls light emitting spectrums by adjusting the anion halides in perovskite materials. The key is to adjust the anion halides by simply dissolving them in solvents to achieve red, blue, and green light. Application of this technique to LEDs can result in crystal-clear picture quality, they say.
Perovskite nanoparticles emit different colours depending on the internal halogen element: red when rich in iodine, green when rich in bromine, and blue when rich in chlorine. However, perovskite is highly sensitive, making it difficult to change elements stably. In search for an answer, Kim has developed a simple technique to replace certain elements via a solution process. This is a method of inducing element substitution, using nonpolar solvent and chemical additives.
"In the study, we added a nonpolar solvent, containing iodine , bromine and chlorine to a solution of perovskite nanoparticles," says Yung Jin Yoon, the first author of the study. "Once the reaction takes place, the elements mixed within the nonpolar solvent switches its place with elements in original perovskite, which causes changes in luminescence.
The added chemical additive serve to separate the halogen element present in the nonpolar solvent. As a result, the amount of halogen element in the solution increases, and over time, it is replaced with a halogen element in the conventional perovskite. The emission colour is determined by the number of elements in the perovskite. The researchers also succeeded in making LEDs with red, blue, and green colours using perovskite nanoparticles made with this technology.
Kim Ki-Hwan, a research professor in the Department of Energy and Chemical Engineering, said: "It is stable compared to the existing technology to change the element in the solid perovskite." "It can be applied variously to change the element composition in the perovskite material, I hope it will be possible. "
"With our simple method, we obtained luminescence covering the entire visible spectrum from 400 to 700 nm," says Kim. "Furthermore, saturated and vivid RGB LED devices were successfully fabricated using the anion-exchanged nanocrystals."


































