A tandem boost to solar-powered water splitting
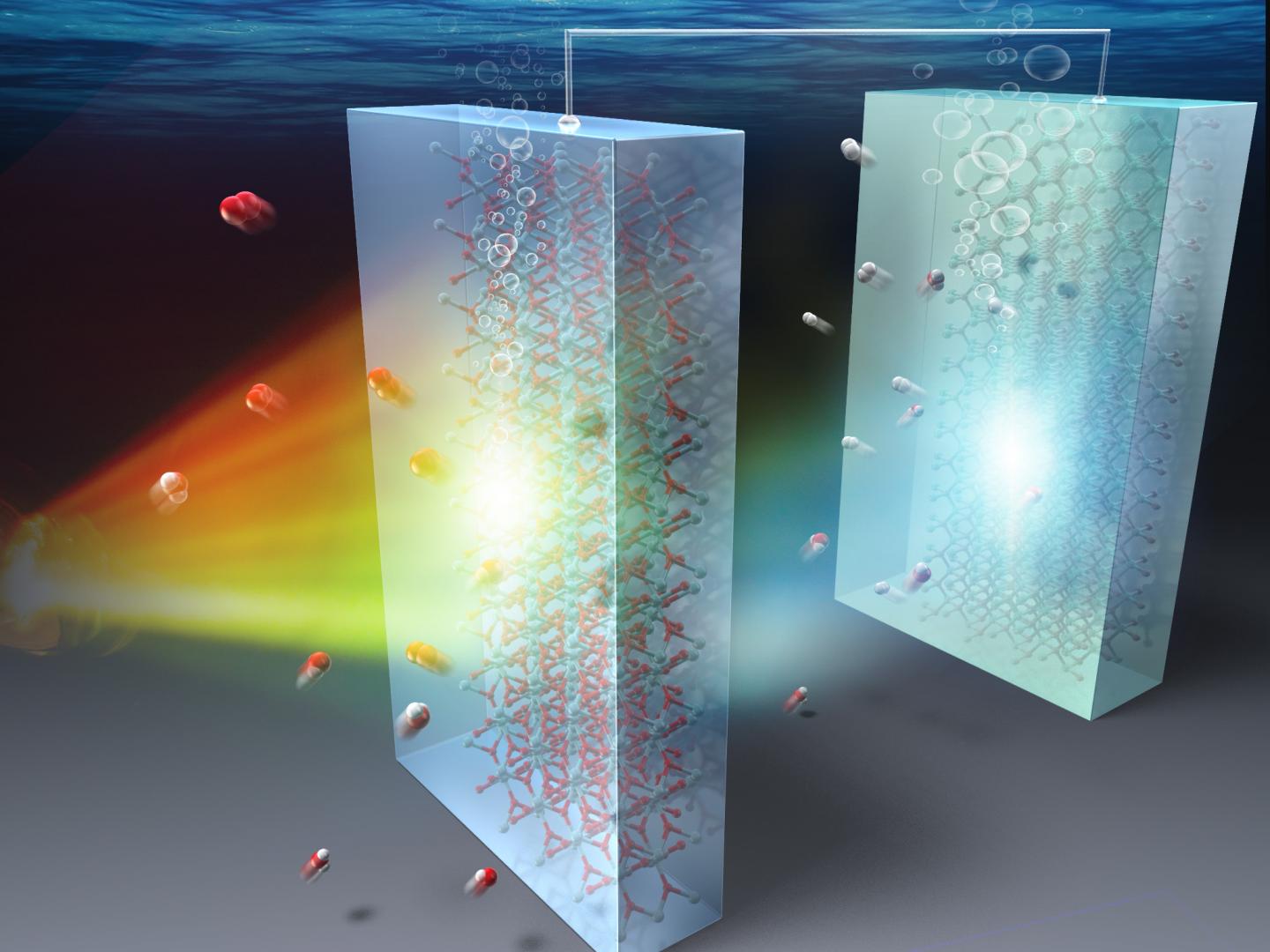
Scientists combine two promising photocatalysts to obtain higher solar-to-hydrogen conversion efficiency and durability in a water splitting cell
Both industry and academia have been focusing heavily on hydrogen as a feasible clean alternative energy source. Hydrogen is practically inexhaustible and when used to generate energy, only produces water vapour. However, to realise a truly eco-friendly hydrogen society, we need to be able to mass-produce hydrogen cleanly in the first place.
One way to do that is by splitting water via 'artificial photosynthesis' with photocatalysts that use solar energy to produce oxygen and hydrogen from water. However, the available photocatalysts are not yet where they need to be to make solar-powered water splitting economically feasible and scalable. To get them there, two main problems should be solved: the low solar-to-hydrogen (STH) conversion efficiency and the insufficient durability of photoelectrochemical water splitting cells.
At Nagoya Institute of Technology, Japan, Masashi Kato and his colleagues have been working hard to take photocatalysts to the next level by exploring new materials and their combinations and gaining insight into the physicochemical mechanisms that underlie their performances. In their latest study published in Solar Energy Materials and Solar Cells, Kato and his team have now managed to do just that by combining TiO2 and p-type cubic SiC (3C-SiC), two promising photocatalyst materials, into a tandem structure that makes for a highly durable and efficient water splitting cell. The figure above shows how a semitransparent TiO2 photoanode allows the SiC photocathode to make use of the transmitted light. Using photocatalysts with different energy gaps results in increased conversion efficiency.
The tandem structure explored by the team in their study has both the photocatalyst materials in series, with a semi-transparent TiO2 operating as a photoanode and 3C-SiC as a photocathode. Since each material absorbs solar energy at different frequency bands, the tandem structure can markedly increase the conversion efficiency of the water splitting cell by allowing more of the incoming light to excite charge carriers and generate the necessary currents.
The team measured the effects of applied external voltage and pH on the photocurrents generated in the cell and then conducted water splitting experiments under different light intensities. They also measured the amounts of oxygen and hydrogen generated. The results were highly encouraging, as Kato remarks: "The maximum applied-bias photon-to-current efficiency measured was 0.74 percent. This value, coupled with the observed durability of about 100 days, puts our water splitting system among the best currently available." Moreover, the findings of this study hinted at some of the potential mechanisms behind the observed performance of the proposed tandem structure.
Further research is needed to continue improving photoelectrochemical water splitting systems until they become widely applicable. Still, this study is clearly a step towards a clean future. "Our contributions shall accelerate the development of artificial photosynthesis technologies, which will generate energy resources directly from solar light. Thus, our findings may assist in the realisation of sustainable societies," says Kato.


































