Scientists re-engineer TiO2 to make green hydrogen
international team finds novel way to extends titanium dioxide's photosensitivity to visible light
A research team from INRS (Institut National de Recherche Scientifique) in Canada, has joined forces with French researchers from ICPEES (a CNRS-University of Strasbourg joint research lab) to pave the way towards the production of green hydrogen. They are using a re-engineered version of the compound semiconductor TiO2.. The results were published in the November 2020 issue of Solar Energy Materials and Solar Cells.
Hydrogen is being considered by several countries of the Organisation for Economic Co-operation and Development (OECD) as a key player in the transition towards decarbonised industries and sectors. According to the INRS professor My Ali El Khakani, Quebec could strategically position itself in this energy sector of the future. "Thanks to high-performance nanomaterials, we can improve the efficiency of water dissociation to produce hydrogen. This 'clean' fuel is becoming increasingly important for the decarbonisation of the heavy-duty trucking and public transportation. For example, buses using hydrogen as a fuel are already in operation in several European countries and in China. These buses emit water instead of greenhouse gases," added the physicist and nanomaterials specialist.
Splitting water molecules into oxygen and hydrogen has long been done by electrolysis. However, industrial electrolyzers are very energy-intensive and require large investments. The INRS and ICPEES researchers were rather inspired by photosynthesis. Indeed, they have developed specially engineered and structured electrodes that split water molecules under the sun's light.
Challenges...
For maximum use of solar energy, the research teams have selected TiO2 , a semiconductor known for being photosensitive to UV-light, which accounts only for 5 percent of the solar irradiance. The teams have used their expertise to first change the atomic composition of TiO2 and extend its photosensitivity to visible light. They were able to produce electrodes that can absorb up to 50 percent of the light emitted by the sun. A significant gain right from the start!
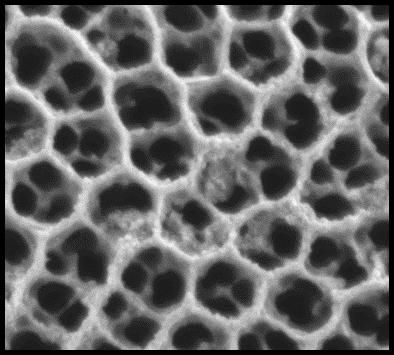
The researchers have then proceeded with the nanostructuration of the electrode to form a network of TiO2 nanotubes that resembles a beehive-like structure. This method multiplied the effective surface area of the electrode by a factor of 100,000 or more. "Nanostructuring maximizes the ratio between surface and volume of a material. For example, TiO2 nanostructures can offer a surface area of up to 50 m2 per gram. That's the surface area of a mid-size flat!", said El Khakani.
The final step of the electrode elaboration is their 'nanodecoration'. This process consists of depositing catalyst nanoparticles on the otherwise infinite network of TiO2 nanotubes to increase their efficiency of hydrogen production. To achieve this nanodecoration step, the researchers used the laser ablation deposition technique, a field where El Khakani has developed expertise in over the last 25 years. The challenge was not only to control the size, dispersion and anchorage of catalyst nanoparticles on the TiO2 nanotube matrix, but also to find alternatives to the costly iridium and platinum classical catalysts.
This research identified cobalt oxide (CoO), a material that is quite available in Quebec's underground, as effective co-catalysts for splitting water molecules. A comparison of the two materials showed that CoO nanoparticles enabled a tenfold increase the photocatalytic efficiency of these new nanodecorated electrodes under visible light compared to bare nanotubes.
The picture above is an electron microscopy image of an array of TiO2 nanotubes (like honeycomb cells of a bee hive) decorated with CoO nanoparticles.
'Comparative study of the photocatalytic effects of pulsed laser deposited CoO and NiO nanoparticles onto TiO2 nanotubes for the photoelectrochemical water splitting' by Thomas Favet et al; Solar Energy Materials and Solar Cells, November 2020