A less toxic way to make metal halide perovskite nanocrystals?
Chinese team uses germanium halides to synthesise Pb-free and Pb-based PNCs
Organohalides are widely adopted to serve as halide source in traditional three-precursors route to obtain metal halide perovskite nanocrystals (PNCs).
However, these organohalides are usually highly toxic, which is unfavourable for large-scale and sustainable use. Moreover, their efficacy in producing high-quality Pb-free PNCs is questionable.
Recently, a research group led by Ke-li Han from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) used all-inorganic nonhazardous GeX4 (X = Cl, Br, I) as a halide source to synthesize both Pb-free and Pb-based PNCs with high optoelectronic quality.
This study was published in Nano Letters on January 12.
They found that Ge element wasn't present in the final compositions, whereas material properties of the resulting NCs were improved, such as stronger photoluminescence emission and enhanced phase stability.
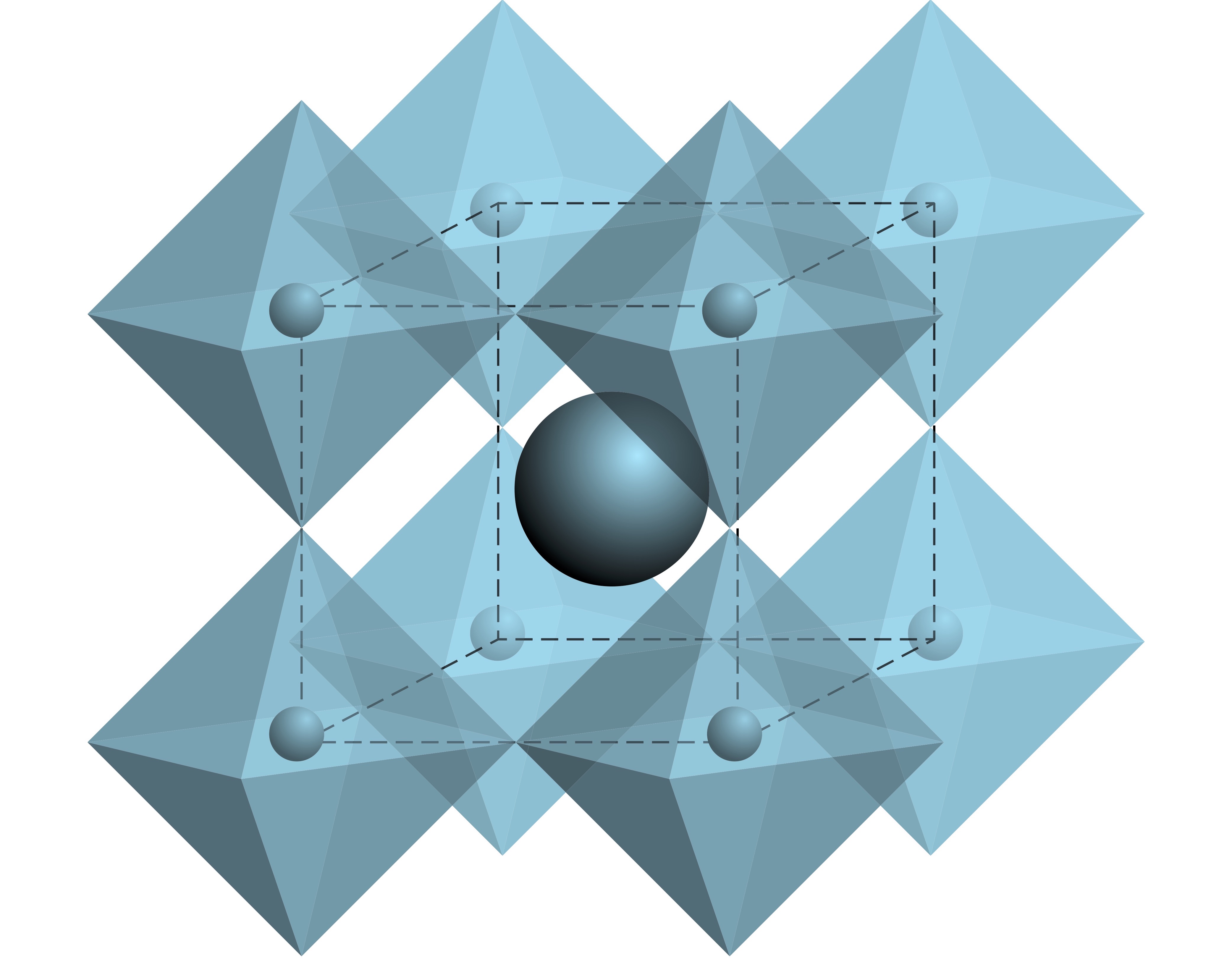
They attributed these improved properties to a better control over the release of halide ions in Ge halide-based route, which helped the PNCs to form a regular crystal surface with less point defects.
Moreover, the advantage of the proposed GeX4 approach in making ideal PNCs lied in their unique dielectric environment and thermodynamics.
"It is foreseen that the GeX4-based synthesis could provide a yet unexplored less toxic path to produce these fascinating nanomaterials and tailor their optical properties without alerting their intrinsic structure," said Han.
REF
'Germanium Halides Serving as Ideal Precursors: Designing a More Effective and Less Toxic Route to High-Optoelectronic-Quality Metal Halide Perovskite Nanocrystals' by Xiaochen Wang et al; Nano Lett. 2022,January 12, 2022