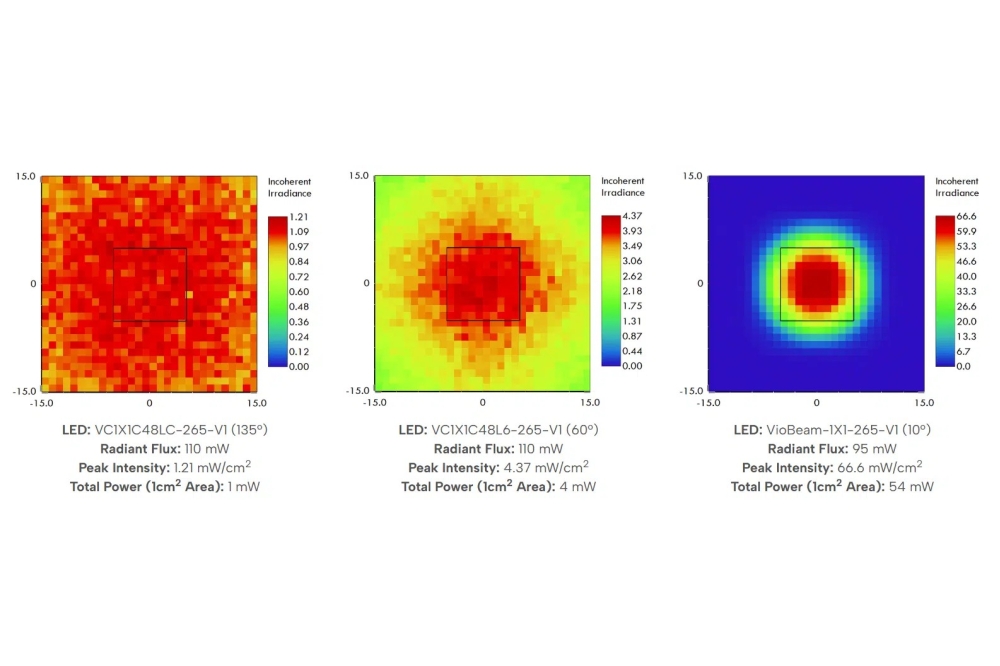
Novel tandem cell combo achieves 25.7% efficiency
Felix Lang from Potsdam University, Germany and colleagues Lei Meng and Yongfang Li from the Chinese Academy of Sciences, Beijing, have combined perovskite and organic solar cells to achieve 25.7 percent efficiency with a low temperature processing technique.
Their work 'Isomeric diammonium passivation for perovskite–organic tandem solar cell' was published in Nature.
Combining two materials that selectively absorb short and long wavelengths is a well-known strategy to increase efficiency in solar cells. The best est red/infrared absorbing parts of solar cells so far, however, have been made from traditional materials, such as silicon or CIGS. Unfortunately, these require high processing temperatures, and thus exhibit a relatively high carbon footprint.
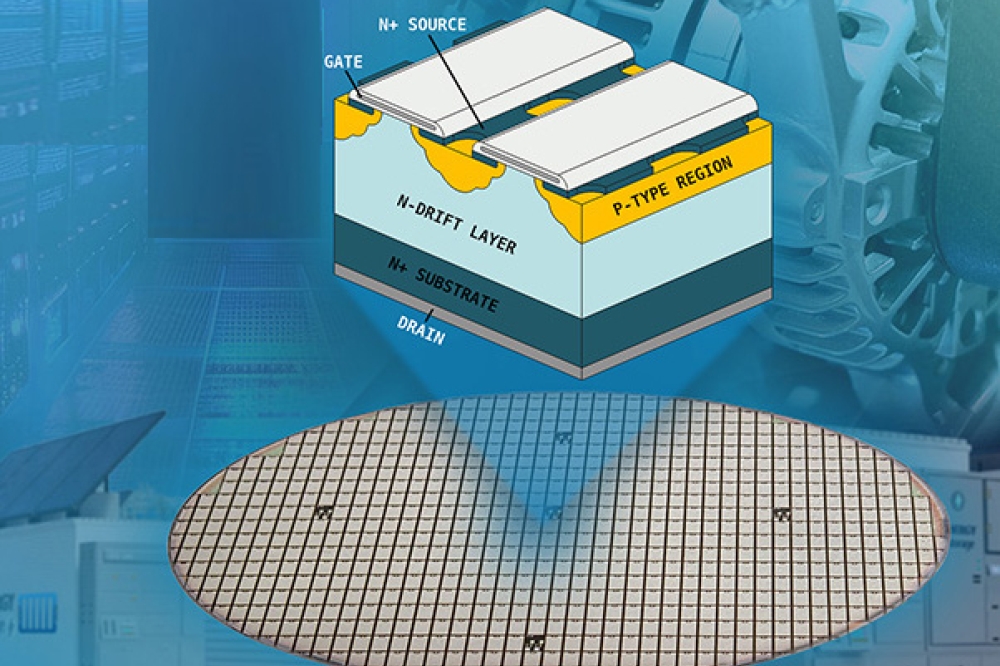
Perovskite–organic tandem cells, comprising a wide-bandgap (WBG) perovskite solar cell as the front cell and a narrow-bandgap organic solar cell (OSC) as the rear cell, have recently drawn attention owing to the good stability and potential high power conversion efficiency (PCE). However, WBG perovskite cells usually exhibit higher voltage losses than regular perovskite cells which limits the performance of the tandem combination.
Lang and colleagues said achieving 25.7 percent efficiency was not easy. “This was only possible by combining two major breakthroughs,” said Lang. First his colleagues Meng and Li synthesised a novel red/infrared absorbing organic solar cell that extends its absorption even further into the infrared.
“Still, tandem solar cells were limited by the perovskite layer, which shows strong efficiency losses if adjusted to absorb only blue/green parts of the sun spectrum”, he explains. “To tackle this, we utilised a novel passivation layer applied to the perovskite that reduces material defects and improves the performance of the whole cell.
The new surface passivator was cyclohexane 1,4-diammonium diiodide (CyDAI2), which naturally contains two isomeric structures with ammonium groups on the same or opposite sides of the hexane ring, and the two isomers demonstrate completely different surface interaction behaviours.