Breakthrough for low-cost solar hydrogen production
A collaborative team of researchers from Imperial College London and Queen Mary University of London has achieved a significant milestone in sustainable energy technology, as detailed in their latest publication in Nature Energy.
The study 'Enhanced Solar Water Oxidation and Unassisted Water Splitting Using Graphite Protected Bulk Heterojunction Organic Photoactive Layers ' shows a pioneering approach to harnessing sunlight for efficient and stable hydrogen production using cost-effective organic materials, potentially transforming the way we generate and store clean energy.
The research tackles a longstanding challenge in the development of solar-to-hydrogen systems: the instability of organic materials such as polymers and small molecules in water and the inefficiencies caused by energy losses at critical interfaces. To address this, the team introduced a multi-layer device architecture that integrates an organic photoactive layer with a protective graphite sheet functionalised with a nickel-iron catalyst. This innovative design achieved an unprecedented combination of high efficiency and durability, setting a new benchmark for the field.
“Our work demonstrates that high-performance, stable solar water splitting can be achieved using low-cost, scalable organic materials,” said Flurin Eisner, Lecturer in Green Energy at Queen Mary University of London, who led the development of the organic photoactive layers during the project.
“Organic materials are highly tunable in terms of their properties, such as the light they absorb and their electrical properties, which means they can be an extremely versatile platform on which to build various ways to convert sunlight into fuels (such as hydrogen) or even chemicals, emulating natural photosynthesis in plants. This opens exciting new avenues for sustainable fuels and chemicals production.”
In the study, the new device achieved a photocurrent density of over 25 mA cm⁻² at +1.23 V vs. the reversible hydrogen electrode for water oxidation – one half of the reaction to split water into hydrogen and oxygen using solar energy. This represents a major leap, surpassing previous systems. Unlike earlier designs that degraded within hours, the new system showed operational stability for days. The design supports a wide range of organic materials, offering flexibility for future innovations in solar energy.
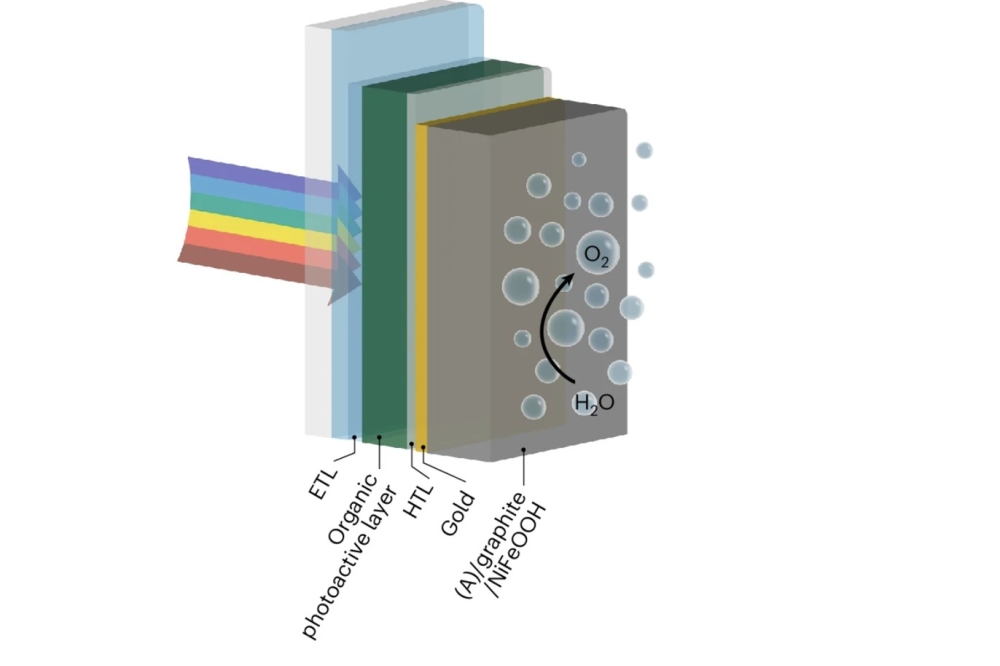
To achieve these results, the team employed a bulk heterojunction organic photoactive layer, integrating a self-adhesive graphite sheet functionalised with an earth-abundant nickel-iron oxyhydroxide catalyst. The graphite not only protected the photoactive layer from water-induced degradation but also maintained efficient electrical connections.
“Beyond the record efficiency and stability of our organic devices, our results disentangle the contribution of the different components in the device degradation, which has been a significant challenge of the field,” said Matyas Daboczi, first author of the study at Imperial’s Department of Chemical Engineering (now Marie Skłodowska-Curie Research Fellow at the HUN-REN Centre for Energy Research and a Visiting Researcher in the Department of Chemical Engineering at Imperial). “I believe that our insights and guidelines will be valuable for further improving the stability and performance of such organic photoelectrochemical devices towards real-world application.”
The potential of this breakthrough was further showcased in full water splitting devices, capable of generating hydrogen from water and light without the need for any additional electricity. They achieved a solar-to-hydrogen efficiency of 5 percent, a feat that could significantly accelerate the adoption of, for example, off-grid hydrogen production technologies.
Salvador Eslava, lead academic of the study at Imperial’s Department of Chemical Engineering, stated: “This result is a significant improvement in organic photoelectrochemical device performance, achieving record solar-to-hydrogen efficiencies. The approach leverages the advantages of organic bulk heterojunctions, which offer impressive photocurrents, photovoltages, abundant elements, and ease of processing, and applies them to the electrodes of photoelectrochemical cells.”
The study's outcomes are expected to spark further advancements in the field, paving the way for real-world applications. The team aims to build on this foundation, exploring improvements in material stability and scaling the technology for industrial use.
Pictured above: A schematic representation of the water oxidation IPV-anodes containing an organic photoactive layer and a self-adhesive (A) graphite sheet as a conductive protection layer (not to scale). The adhesive layer is not shown, as there is direct electrical contact between the gold and graphite layers.