High-volume production of AlInGaN-based LEDs
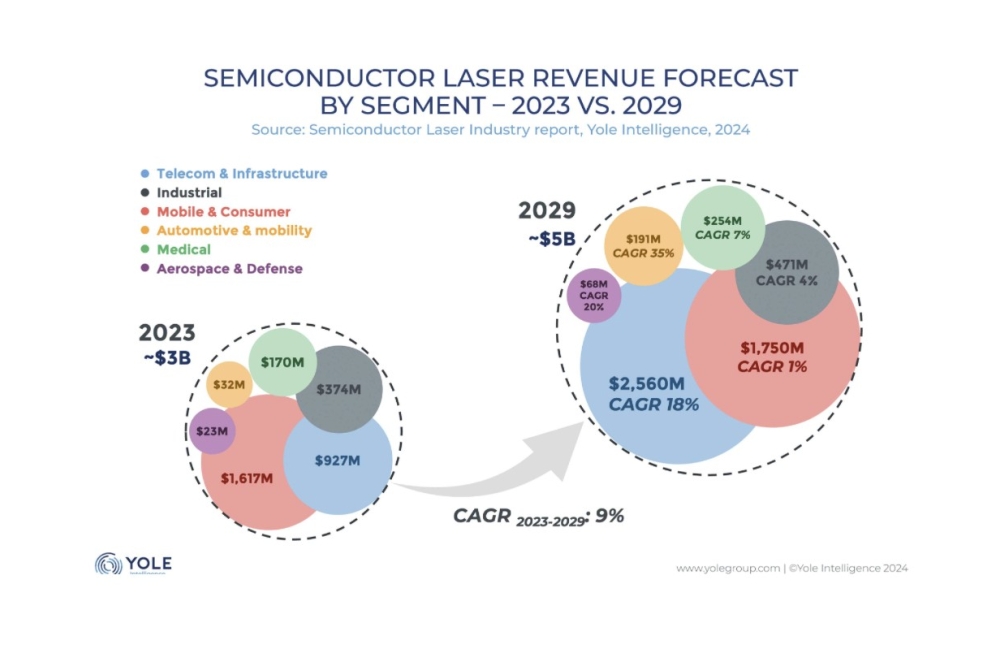
During the last seven years the market for AlInGaN-based LEDs has grown from almost zero to about $600 million in 2000. It is predicted that the market will grow at a compound annual growth rate of about 20% until the end of the decade (Strategies Unlimited). However, this forecast does not take into account the general lighting market as it is expected that LEDs will not be able to compete with conventional lighting until the next decade due to cost and performance. General lighting is considered the holy grail of the high-brightness LED industry since it represents a much larger market size of many billions of dollars. With such tremendous market opportunity in both the near and long terms, a huge investment has been made in building up the manufacturing capacity for these devices. Compared to other existing LED growth technologies such as LPE, VPE of GaP-based LEDs, and MOCVD of AlInGaP-based LEDs, the nitride LED manufacturing process is much less mature and the cost of making blue and green LEDs is much higher. As with any other technology in the early stages of its evolution, there are issues which need to be addressed before cost-effective manufacturing can be realized. Manufacturing issues LEDs are a so-called penny business. Conventional LEDs sell at sub-penny levels per packaged lamp, high-end AlInGaN-based LEDs can sell at more than 50 cents. Compared to laser diodes, which usually sell in the few dollars range, LEDs are much more cost-sensitive components to fabricate. The overall yield for conventional types of LEDs (e.g. AlGaAs-based) is normally better than 80%, and the throughput in the manufacturing process is much greater than those utilizing MOCVD processes. In recent years, however, high-throughput MOCVD reactors (more than 35 x 2 inch wafers per run) for the production of AlInGaP-based high-brightness LEDs (HB-LEDs) are gaining acceptance in the industry. It took about a decade for the low-cost manufacturing process to be developed for AlInGaP-based HB-LEDs. Many of the challenges that have been resolved are related to MOCVD processes such as epilayer structure, uniformity and defect control. Nevertheless, the production of AlInGaP-based HB-LEDs is still more expensive than that of conventional devices. The general consensus is that AlInGaN-based LEDs will follow a similar evolutionary path to that of AlInGaP-based HB-LEDs. One example of this is that since the introduction of AlInGaN-based HB-LEDs in 1994 by Nichia, the production MOCVD process has evolved from using single wafers to being a multi-wafer batch process. Whether this analogy will continue in the next few years, however, will depend on how some specific issues are resolved. In our view, there are two major issues specific to the AlInGaN material system that have a major impact on the manufacturing cost. One is the color uniformity control in the MOCVD process, and the other is the dicing of AlInGaN-on-sapphire wafers, which is much harder than the dicing of AlInGaP-on-GaAs wafers.
The manufacturing of AlInGaN-based LED chips involves three steps: MOCVD growth, wafer fabrication and die separation. The level of LED chip performance and consistency is usually dictated by the applications in which the devices are used. For example, in outdoor full-color video LED displays, both brightness and uniformity are important characteristics in achieving good picture quality. The required color uniformity is very tight, roughly 5 nm for blue and 2 nm for green. This is a challenge for low-cost manufacturing due to the fact that current MOCVD processes can only achieve poor uniformity. This is also true for cyan LEDs for traffic lights, and blue LEDs for phosphor down-conversion to create white light. MOCVD epitaxial process uniformity In the growth of AlInGaN thin films on sapphire by MOCVD, ammonia (NH3) is the most commonly used nitrogen source. Due to the low decomposition efficiency of NH3 at growth temperatures, the required partial pressure of NH3 in the reaction chamber is much higher than that required for AsH3 or PH3 during the growth of As- or P-based compounds. The required NH3 flow rate is therefore so high that the gas properties of NH3 have to be considered in the overall flow dynamics during the growth process. This is not the case for processes using AsH3 or PH3, in which the concentration of hydride is much lower, and where the overall flow dynamics are determined solely by the property of the main carrier gas, such as hydrogen or nitrogen. Since NH3 is a relatively viscous gas, it is difficult to maintain a good laminar flow. Usually a very high total flow rate and a small reactor geometry are used. One example is the two-flow reactor developed by Nichia (Nakamura et al.). On the other hand, to achieve good uniformity in a large area, a low growth pressure has to be used to avoid the need for unrealistically high gas flow rates. However, as mentioned previously in the MOCVD growth of AlInGaN compounds, a large NH3 partial pressure is required due to the low decomposition efficiency of NH3. The total growth pressure therefore cannot be too low in order to maintain sufficient NH3 partial pressure, and this in turn requires a trade-off for the flow dynamics, a low-pressure condition to achieve a high degree of laminar flow. Figure 1 shows a trend chart of color uniformity for a production period of three months; samples were grown in a multi-wafer commercial reactor. The target wavelength is 470 nm. Each bar represents the variation within a wafer and is about 10 nm. We also calculated the variation of the average wavelength from each wafer, which was about 15 nm. This means the overall maximum variation could be as high as 25 nm.
Compared to the MOCVD production of AlInGaP-based LEDs, the wavelength control in this case is much worse. Aside from flow dynamics, it is believed that the other cause of variation is indium segregation (Narukawa et al.). The mechanism is even more severe at longer wavelengths as more indium is required. How the process parameters in the MOCVD process correlate to the indium segregation mechanism is not clear. However, we do know that temperature and pressure are usually critical factors in determining the chemistry and kinetics of the indium complex on the surface. More research is needed to further understand the mechanism and to develop engineering techniques to control this segregation process.