Quantum dots: from the visible to the infrared
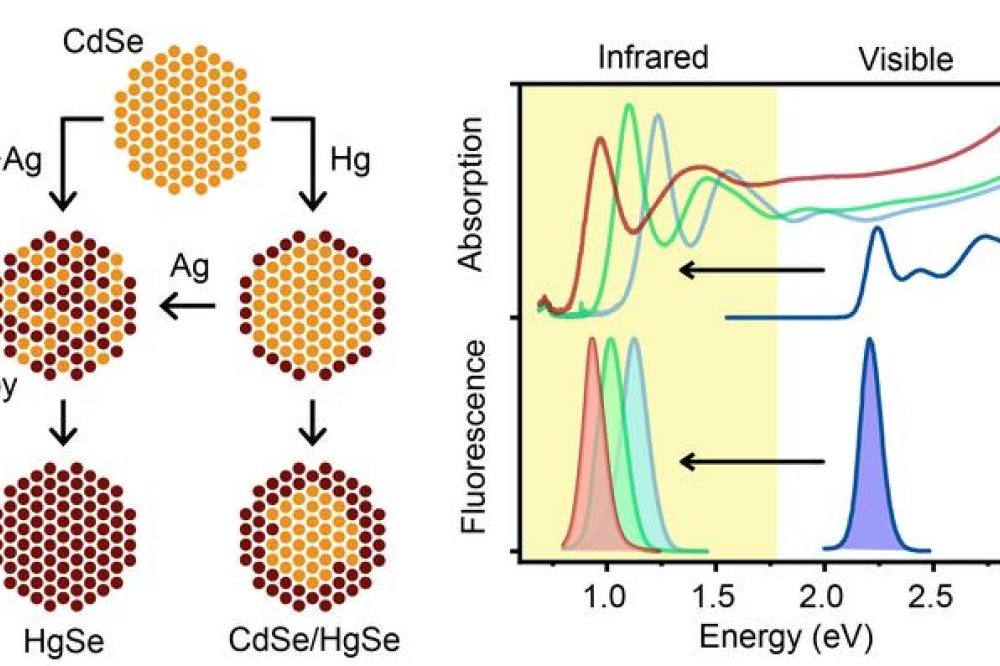
In new research published in Nature Synthesis, University of Illinois at Urbana-Champaign bioengineering professor Andrew Smith and postdoctoral researcher Wonseok Lee have developed HgSe and HgCdSe quantum dots that absorb and emit in the infrared, made from already well-developed, visible spectrum CdSe precursors.
The new nanocrystal products retained the desired properties of the parent CdSe nanocrystals, including size, shape and uniformity.
“This is the first example of infrared quantum dots that are at the same level of quality as the ones that are in the visible spectrum,” Smith says.
Smith had been trying to make this material since he was in graduate school with no luck, and even in the broader research community, there have been no reports of success, until now. “The way we did it was taking the already perfected, visible ones—cadmium selenide, which is considered to be the most developed quantum dot—and used it as a ‘sacrificial mold’,” he says.
Replacing the cadmium atoms with mercury atoms instantly shifts everything into the infrared spectrum, with all the desired quality retained: strong light absorption, strong light emission and homogeneity.
Described in the Nature Synthesis paper 'Interdiffusion-enhanced cation exchange for HgSe and HgCdSe nanocrystals with infrared bandgap' Smith and Lee achieved this by ditching the traditional method of synthesis for nanocrystals, which is to mix the precursor elements together and under the right conditions, they decompose into the desired nanocrystal form. As it turns out, there are no conditions that anybody has found to work for mercury, cadmium and selenide.
“Lee developed a new process called interdiffusion enhanced cation exchange,” Smith says. “In this process, we add a fourth element, silver, which introduces defects in the material that causes everything to mix together homogeneously. And that solved the whole problem.”
While quantum dots have many applications, one application for infrared quantum dots with potential to have the most impact is for use as molecular probes for imaging, where they can be put into biological systems and detected in tissues. Since most quantum dots emit in the visible spectrum, only emission near the surface of the skin can be detected. Biology, however, is fairly transparent in the infrared and therefore deeper tissues can be probed.
Mice are the standard models for most diseases and Smith explains that with quantum dots that emit in the infrared, researchers could see almost entirely through a living rodent to see its physiology and the locations of specific molecules throughout the body.
This will allow for better understanding of biological processes and for developing therapeutics without having to sacrifice the mice, potentially changing preclinical drug development.
This research was funded by the US National Institutes of Health and the National Science Foundation.